
Good News for Patients with Blood Cancers! World’s Third BCL-2 Inhibitor——Sonrotoclax Approved in China
On January 6, 2026, BeiGene (Suzhou) Biotech Co., Ltd. (BeiGene) ‘s independently developed innovative drug Sonrotoclax tablets (trade name: Baiyueda®) were officially approved for market launch in China.
The approved indications for sonrotoclax tablets in China encompass two patient populations:🔽
👉 Adult patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) who have received at least one prior systemic therapy, including a Bruton’s tyrosine kinase (BTK) inhibitor;
👉 Adult patients with relapsed or refractory mantle cell lymphoma (MCL) who have received at least two prior systemic therapies, including a BTK inhibitor.

Sonrotoclax Tablets represent China’s second domestically developed BCL-2 inhibitor and the world’s third drug of its kind.
This approval marks another significant breakthrough in China’s innovation in hematological oncology drug development.
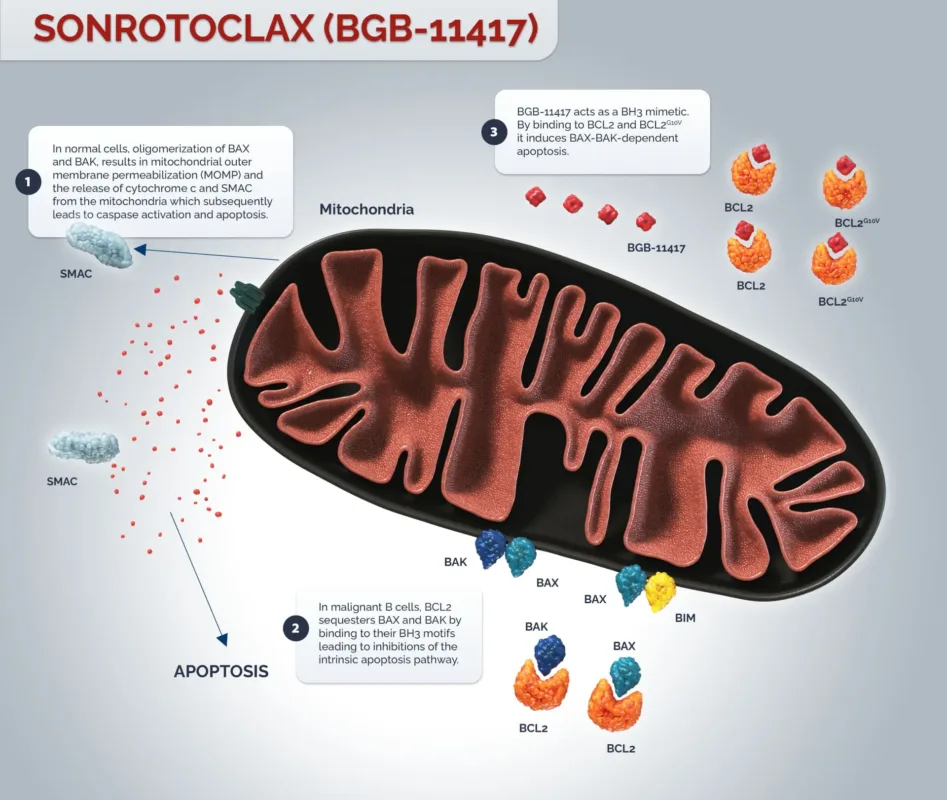
Sonrotoclax Mechanism of Action—A novel, Potent, and Highly Selective BCL-2 Inhibitor
The BCL-2 protein family serves as a key regulator of apoptosis. In various B-cell malignancies, overexpression of BCL-2 proteins suppresses programmed cell death in tumor cells, leading to abnormal survival and the development of drug resistance.
Compared to traditional BCL-2 inhibitors, Sonrotoclax incorporates multiple molecular design optimizations. It precisely binds to the BH3 domain of BCL-2 proteins, blocking their interaction with pro-apoptotic proteins and thereby restoring the apoptotic pathway in tumor cells.
Sonrotoclax’s most distinctive pharmacokinetic feature is its short half-life and non-accumulative properties, which hold significant clinical value for combination therapy and individualized dose adjustment.
✨The short half-life enables rapid drug clearance, allowing timely discontinuation or dose reduction upon adverse reactions and reducing the risk of severe toxicity.
✨The non-accumulative property ensures long-term treatment safety by preventing potential organ damage caused by sustained drug accumulation in the body.
Sonrotoclax Clinical Trials: Reliable Data Support
Sonrotoclax approved in China is based on positive results from two pivotal clinical trial.
1️⃣ In the BGB-11417-202 study for CLL/SLL, 100 patients with relapsed or refractory disease who had received prior intensive therapy were enrolled.
Median follow-up of 14.4 months revealed an overall response rate of 76% for sonrotoclax monotherapy, as assessed by an independent review committee, including a complete response or complete response with incomplete hematopoietic recovery rate of 19%.
2️⃣ In the BGB-11417-201 study for MCL, the drug also demonstrated significant efficacy. This global multicenter study enrolled 103 patients with relapsed or refractory mantle cell lymphoma who had previously received anti-CD20 therapy and BTK inhibitor treatment.
Results showed an overall response rate of 52.4% and a complete response rate of 15.5%, as assessed by an independent review committee.
Notably, the overall response rate remained as high as 59.1% in the high-risk subgroup with TP53 mutations, indicating potent activity even against the most aggressive subtype of MCL.
Therapeutic Landscape: Addressing Multiple Gaps in Hematologic Oncology Treatment
😢 For patients with CLL/SLL, a major clinical challenge lies in the severely limited options for effective subsequent treatment once disease progression occurs following standard regimens represented by BTK inhibitors.
✅ Sonrotoclax tablets, as a novel BCL-2 inhibitor with a distinct mechanism of action, provide an important sequential treatment option for CLL/SLL patients.
😢The value of this drug is even more pronounced in the treatment of the more aggressive MCL.The vast majority of MCL patients eventually develop resistance to initial therapy, progressing to a relapsed or refractory state.
Particularly for patients who develop resistance to BTK inhibitors, the prognosis deteriorates significantly, with median survival typically less than one year, creating an urgent and unmet clinical need.
✅Key clinical data demonstrate that sonrotoclax achieves an overall response rate exceeding 50% in patients with relapsed or refractory MCL who have undergone multiple prior lines of therapy, offering renewed hope for this high-risk patient cohort.
Consequently, Sonrotoclax tablets not only provide a novel therapeutic option for specific patient populations, but more importantly, transform the treatment landscape for CLL/SLL and MCL, offering a clearly effective solution for overcoming BTK inhibitor resistance.
Global Perspective: Sonrotoclax has the Potential to Become the First BCL-2 Inhibitor Approved in the U.S. for R/R MCL
Sonrotoclax company not only focuses on the Chinese market but also possesses a global outlook. It has received Breakthrough Therapy designation from the US Food and Drug Administration (FDA) and priority review status for its marketing application.
This drug is intended for treating adult patients with relapsed or refractory MCL who have received prior treatment with BTK inhibitors. It has the potential to become the first BCL-2 inhibitor approved in the United States for treating relapsed or refractory MCL.
Within the global competitive landscape for BCL-2 inhibitors, only AbbVie’s Venetoclax and Ascentage Pharma’s Lisaftoclax are currently approved for marketing.
👏 As a next-generation product, Baiyueda sonrotoclax‘s differentiated advantages primarily lie in its pharmacokinetic profile and broader indication scope.
As a globally specialised pharmaceutical import-export service provider, DengYueMed efficiently delivers breakthrough medicines, including Sonrotoclax tablets, to more patients in need worldwide, fulfilling its mission to accelerate access to innovative therapies.



