SOTYKTU (Deucravacitinib Tablets) – Psoriasis | HongKong DengYue Medicine
- Generic Name/Brand Name: Deucravacitinib/SOTYKTU
- Indications: Plaque psoriasis, systemic therapy, phototherapy
- Dosage Form: Oral tablet
- Specification: 6mg x 7 tablets
Deucravacitinib Tablets Application Scope
Deucravacitinib is used primarily in the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It may also be used for other autoimmune conditions as prescribed by a doctor.
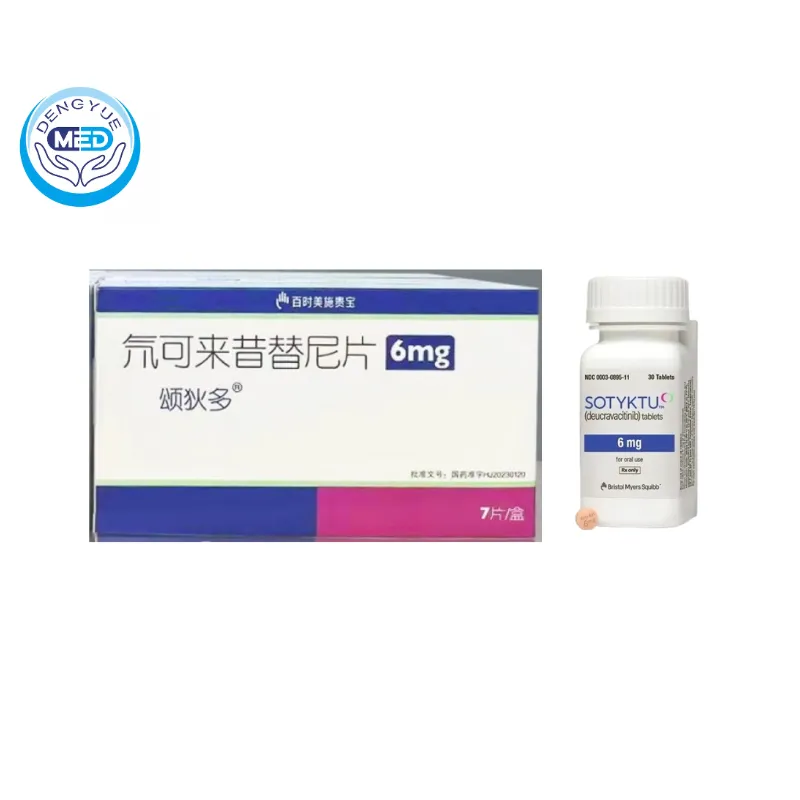
Deucravacitinib Tablets Characteristics
-
Ingredients: Deucravacitinib (active ingredient), inactive ingredients such as fillers, binders, and coating agents.
-
Properties: Deucravacitinib is a selective tyrosine kinase 2 (TYK2) inhibitor that modulates immune responses.
-
Packaging Specification: Typically available in tablet form (e.g., 6 mg tablets)
-
Storage: Store at room temperature, away from moisture and heat.
-
Expiry Date: Refer to packaging for specific expiration.
-
Executive Standard: Should be specified by the manufacturer or regulatory authorities (e.g., GMP standards).
-
Approval Number: Please check the relevant drug regulatory authority (FDA, EMA, etc.) for the approval number.
-
Date of Revision: As per the manufacturer, update when necessary.
-
Manufacturer: SOTYKTU
Guidelines for the Use of Deucravacitinib Tablets
-
Dosage and Administration:
-
Recommended Dose: 6 mg orally once daily, with or without food. Swallow tablets whole; do not crush/cut/chew.
-
Administration: Oral. No routine lab monitoring is mandated in the US label, but see Precautions.
-
Missed Dose: Take the missed dose as soon as remembered unless it is close to the next dose; do not double the next dose. (Per FDA Patient Information.)
-
-
Adverse Reactions:
-
Common Adverse Reactions: Upper respiratory infections, herpes simplex, folliculitis, mouth ulcers, acne, increased CPK levels.
-
Serious Adverse Reactions: Serious infections, malignancy, hypersensitivity reactions, elevated liver enzymes, myopathy.
-
-
Contraindications: Known hypersensitivity to deucravacitinib or any component. (No other absolute contraindications listed in US PI.)
-
Precautions: Screen for TB; avoid live vaccines; consider infection risk; monitor clinically for hepatic or muscular symptoms as indicated; use is not recommended in severe hepatic impairment (Child-Pugh C); pregnancy/lactation per local guidance.
Deucravacitinib Tablets Interactions
-
Immunosuppressants: Concurrent use with other potent immunosuppressants is not recommended.
-
Vaccines: Avoid live vaccines during treatment.
-
CYP/Transporters & pH agents: No clinically significant interactions with common drugs like fluvoxamine, ritonavir, famotidine, rosuvastatin, or methotrexate; no dose adjustment required.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.