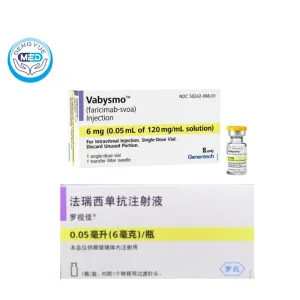
Vabysmo (Faricimab-svoa) – Retinal Vascular Diseases | HongKong DengYue Medicine
- Generic Name/Brand Name: Faricimab-svoa/Vabysmo
- Indications: nAMD, DME, RVO
- Dosage Form: Intravitreal injection solution
- Specification: 6 mg/0.05 mL (120 mg/mL solution) x 1 vial
Faricimab-svoa Application Scope
Vabysmo is indicated for the treatment of:
-
Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
-
Diabetic Macular Edema (DME)
-
Macular Edema Following Retinal Vein Occlusion (RVO)
Faricimab-svoa Characteristics
-
Ingredients: Faricimab-svoa
-
Properties: Sterile, preservative-free, clear to opalescent, colorless to brownish-yellow intravitreal solution
-
Packaging Specification: Single-dose prefilled syringe (6 mg/0.05 mL), single-dose glass vial (6 mg/0.05 mL)
-
Storage: Store refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze. Keep in original carton to protect from light.
-
Expiry Date: Shelf life (as registered): Vial: 30 months; Prefilled syringe: 2 years (check local carton for the printed expiry date)
-
Executive Standard: Approved by regulatory agencies in multiple regions; in the U.S. marketed under BLA BLA 761235
-
Approval Number: U.S. (BLA): BLA 761235 (approved Jan 28, 2022)
-
Date of Revision: The latest FDA approval referenced in searches was for a supplemental application on July 3, 2024
-
Manufacturer: Genentech, Inc./Roche (Roche group)
Guidelines for the Use of Faricimab-svoa
-
Dosage and Administration:
-
Recommended Dose: 6 mg (0.05 mL) per injection
-
Administration:
-
For intravitreal injection only.
-
The procedure must be performed under aseptic conditions, which include using sterile gloves, antiseptic for the skin, and an eyelid speculum
-
-
Missed Dose: If a dose is missed, administer the next dose as soon as possible, and resume the regular schedule
-
-
Adverse Reactions:
-
Common Adverse Reactions: Include conjunctival hemorrhage (bleeding at the white of the eye), eye pain, cataract, and increased intraocular pressure.
-
Serious Adverse Reactions: Include endophthalmitis (a serious eye infection), retinal detachment, and arterial thromboembolic events.
-
-
Contraindications:
-
Patients with ocular or periocular infections.
-
Patients with active intraocular inflammation.
-
Known hypersensitivity to faricimab or any of its components.
-
-
Precautions:
-
Intraocular Pressure Increase: Transient increases in intraocular pressure (IOP) can occur after the injection. Monitor IOP and the perfusion of the optic nerve head.
-
Endophthalmitis: May occur following any intravitreal injection. Patients should be instructed to report any symptoms suggestive of endophthalmitis (e.g., eye pain, increased redness, photophobia, vision blurring) immediately.
-
Retinal Detachment: Monitor for signs of retinal detachment.
-
Thromboembolic Events: Although rare, arterial thromboembolic events have been reported following intravitreal injection of VEGF inhibitors.
-
Special Populations: Use in pregnancy and lactation should only be considered if the potential benefit justifies the potential risk to the fetus or infant.
-
Faricimab-svoa Interactions
- No formal drug–drug interaction studies have been performed. Based on pharmacokinetics and low systemic exposure after ocular administration, no interactions are expected, but VABYSMO should not be administered concurrently with other systemic or ocular anti-VEGF agents. Refer to local product monograph for pregnancy/lactation guidance.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.