China’s First Homegrown ADC for HER2-Mutant NSCLC Approved—Trastuzumab Rezetecan Injection Officially Launched
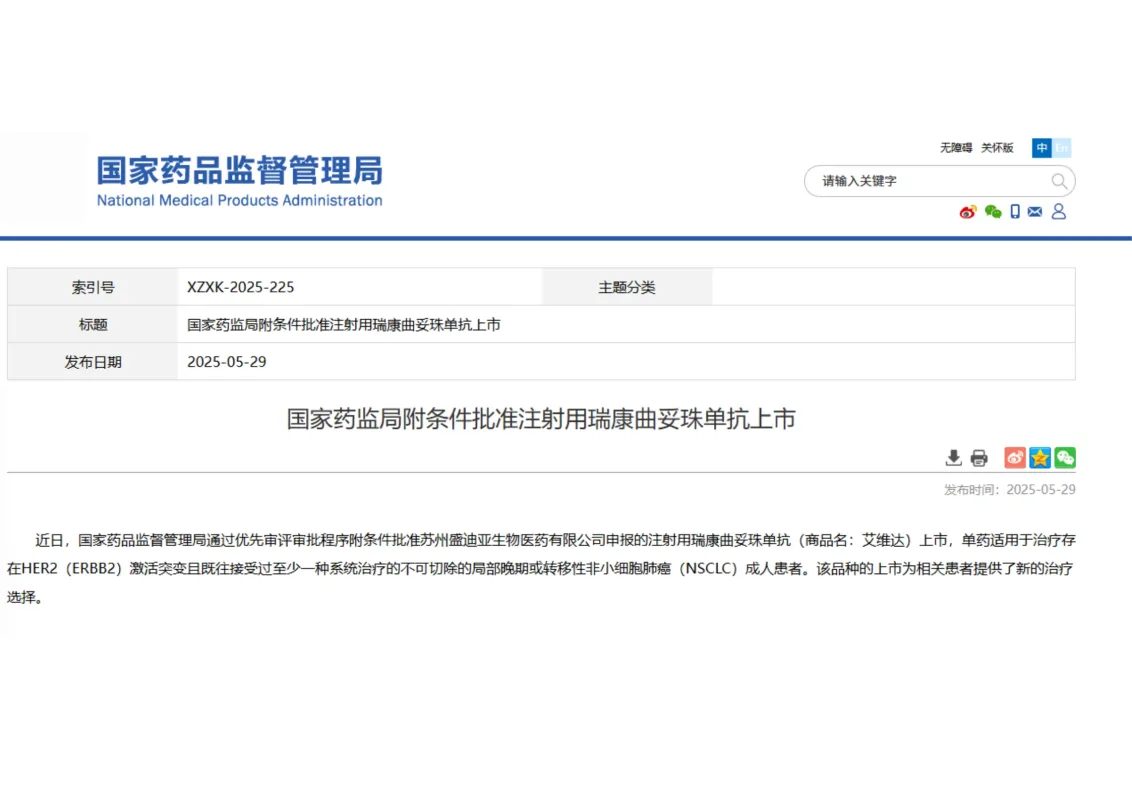
The National Medical Products Administration (NMPA) has officially approved the Class 1 innovative drug Trastuzumab Rezetecan Injection (brand name Aiweida).
The drug was independently developed by Suzhou Suncadia Biopharma Co., Ltd.
👉 It is approved for the treatment of adult patients with unresectable locally advanced or metastatic non-small cell lung cancer (NSCLC).
These patients harbor HER2 (ERBB2) activating mutations. They must have received at least one prior systemic therapy.
✨ This marks the first China-developed antibody–drug conjugate (ADC) approved for HER2-mutant NSCLC, providing a new therapeutic option for patients with limited treatment choices.
Backed by the Pivotal HORIZON-Lung Study: Record-Breaking Global Results
The approval is based on the pivotal HORIZON-Lung study led by Professor Lu Shun from Shanghai Chest Hospital.
✅ Latest study results show:
- An objective response rate (ORR) of 74.5%, as assessed by an independent review committee (IRC), setting a new global benchmark in HER2-mutant NSCLC studies;
- A median progression-free survival (mPFS) of 11.5 months, nearly double that of traditional therapies;
- Significantly reduced toxicity risks compared with conventional ADCs;
- A median follow-up duration of 14.2 months.
The findings were published in The Lancet Oncology in February 2025 and highlighted with updated data at the 2025 AACR Annual Meeting, drawing global attention.
Professor Lu commented, “The study confirms the clinical value of trastuzumab deruxtecan injection in HER2-mutant NSCLC and fills a longstanding treatment gap in China.”
A Decade of Innovation: Hengrui’s ADC R&D Platform Fully Established
👉 The development of trastuzumab rezetecan is supported by Hengrui Pharma’s decade-long establishment of its ADC technology platform.
Powered by its proprietary “Hengrui Swift” modular platform, the company has achieved end-to-end capabilities from molecular design to clinical translation.
Hengrui Pharma President Feng Ji noted that the approval significantly improves treatment accessibility for HER2-mutant NSCLC patients and will further advance China’s precision oncology landscape.

Trastuzumab Rezetecan Injection—Expanding Across Multiple Tumor Types
🧑🔬 Beyond HER2-mutant NSCLC, Trastuzumab Rezetecan injection is advancing across multiple tumor types.
🔻 It has received NMPA Breakthrough Therapy Designations for the following indications:
- Breast cancer
- Colorectal cancer
- Gastric or gastroesophageal junction adenocarcinoma
- Biliary tract cancer
- Epithelial ovarian cancer
- Cervical cancer
- Fallopian tube cancer
- Primary peritoneal cancer
Its potential in HER2-amplified and HER2-overexpressing populations is also under active investigation.
Significance: Accelerating China’s Rise in Global ADC Innovation
💡 The approval of Trastuzumab Rezetecan injection not only addresses the unmet clinical need in HER2-mutant NSCLC in China but also signifies major progress in the nation’s ADC innovation capabilities.
Its launch will accelerate China’s presence in global precision lung cancer therapy and ADC development, ultimately benefiting a broader population of patients.
As an international pharmaceutical distributor, DengYue Medicine continues to follow major breakthroughs in oncology therapies.
💁♂️ If you’d like to gain a deeper understanding of China’s innovative drugs, you can read this article: China Pharmaceutical Innovation: A Golden Decade. After reading it, I believe you will have greater insight into—and trust in—China’s innovative pharmaceutical industry.”🔻