
Mounjaro Breakthrough In Diabetes Treatment With Launch Of Glucose-Lowering Drug Mounjaro
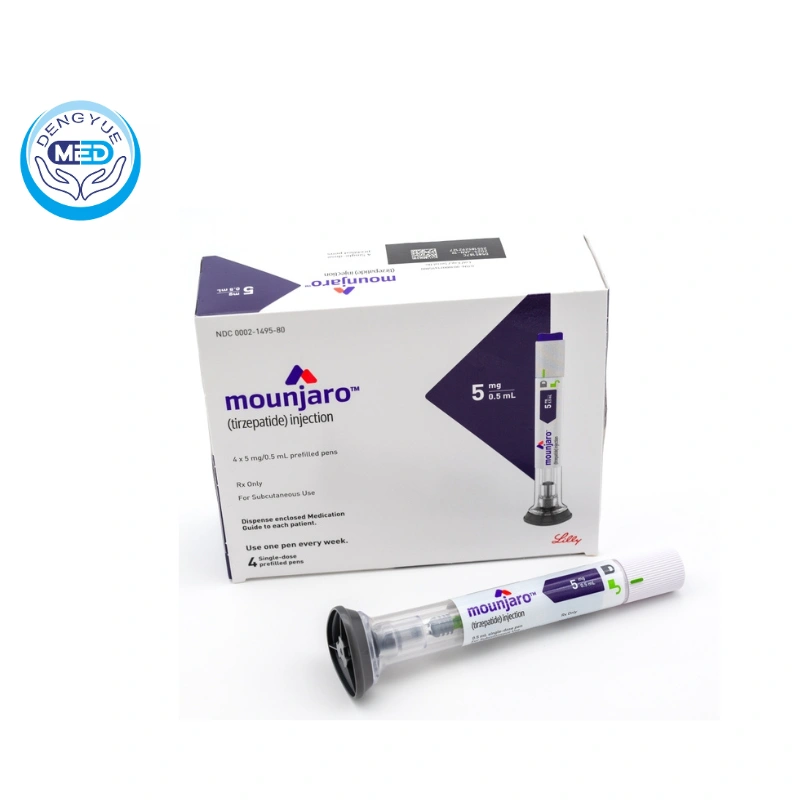
The U.S. Food and Drug Administration (FDA) recently approved the marketing of Mounjaro® (Tirzepatide), a new hypoglycemic drug developed by Eli Lilly and Company (Eli Lilly).
As the first GLP-1/GIP dual agonist, this drug provides a more efficient and safer treatment option for patients with type 2 diabetes mellitus (T2DM).
Revolutionary Mechanism: Dual Targeting For More Potent Glycemic Control
Mounjaro (Tirzepatide) is a once-weekly subcutaneous injection that uniquely targets both glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors.
Which not only significantly reduces blood glucose, but also facilitates weight loss, and even outperforms the mainstream GLP-1 agonists currently available on the market (e.g., Simegle Lutide).
Stunning Clinical Data: Dual Benefits Of Glucose Reduction And Weight Loss
In the pivotal SURPASS series of clinical trials, Mounjaro® demonstrated excellent efficacy:
Glycemic effect: At the highest dose (15mg), patients’ glycated hemoglobin (HbA1c) was reduced by an average of 2.3%, and some patients even achieved diabetic remission.
Weight loss effect: Non-diabetic obese patients in the SURMOUNT-1 trial experienced an average weight loss of 22.5% (about 24 kg), far beyond the traditional weight loss drugs.
Mounjaro Indications And Usage
Mounjaro® is currently approved for use in adults with type 2 diabetes mellitus in conjunction with diet and exercise.
The recommended dosage starts at 2.5mg and gradually increases to 5mg, 10 mg, or 15mg, depending on the patient’s condition.
Safety And Precautions
Common side effects include nausea, diarrhea, and decreased appetite, which are usually mild to moderate and tolerable.
However, be aware of the risk of pancreatitis, gallbladder disease, and C-cell tumors of the thyroid (although rare).
Expert Comment
“The launch of Mounjaro marks a new era in diabetes treatment,” said Dr. John Smith, director of the Diabetes Research Center at Harvard Medical School. ”
Its dual mechanism of action not only provides better glycemic control, but also significantly improves metabolic syndrome problems such as obesity, which is a major boon for patients”.
Looking Ahead
With the accumulation of more clinical data, Mounjaro is expected to become a “bombshell” drug in the field of metabolic diseases.
Lilly has already submitted a marketing application to China’s National Medicines Control Administration (NMPA) and is expected to receive approval in China in 2024.
About DengYueMed – HK Drug Wholesale Distributor
As a legally compliant drug import and export company, DengYueMed is certified by the pharmacy & poisons board of Hong Kong — you can verify our qualification on their official website.

Our efforts to improve the affordability of Tirzepatide drug aim to ensure that more patients can benefit from this important medication.
HK DengYue provides detailed medicine information, transparent pricing, and responsive support to ensure a smooth and reliable buying experience.
Feel free to reach out anytime to discuss your needs or ask questions about the medicine. We welcome you to contact us for a consultation.



