Lazertinib | Non-Small Cell Lung Cancer | HongKong DengYue Medicine
- Generic Name/Brand Name: Lazertinib/Lazcluze
- Indications: Non-Small Cell Lung Cancer
- Dosage Form: Film-coated oral tablets
- Specification: Available in 80 mg and 240 mg strengths.
Lazertinib Application Scope
Lazertinib is indicated for the first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test. It is administered in combination with amivantamab until disease progression or unacceptable toxicity occurs.
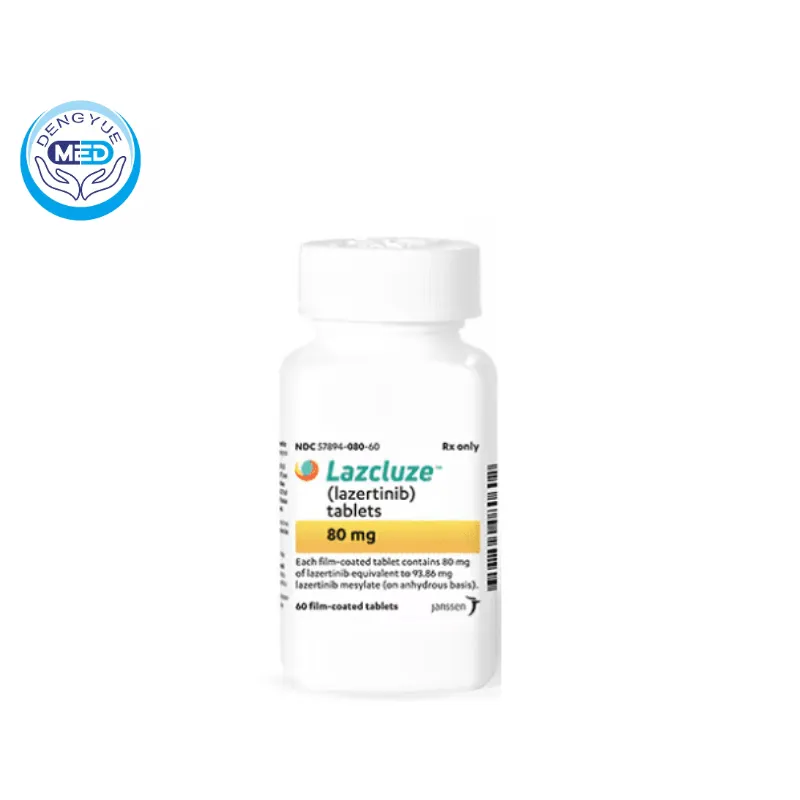
Lazertinib Characteristics
Ingredients:
Active ingredient: Lazertinib mesylate monohydrate
Chemical name: (3S)-N-(1-Cyclopropylethyl)-6,7-dimethoxy-3-(4-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)phenyl)-4-oxo-3,4-dihydroquinoline-2-carboxamide mesylate monohydrate
Properties:
-
Mechanism of Action: Third-generation EGFR tyrosine kinase inhibitor (TKI) targeting EGFR mutations, including T790M resistance mutations.
-
Pharmacokinetics:
-
Absorption: Median time to peak plasma concentration is 2 to 4 hours.
-
Distribution: Plasma protein binding is approximately 99.2%.
-
Elimination: Primarily excreted via feces (86%), with minimal unchanged drug in urine (<0.2%).
-
Half-life: Approximately 3.7 days .
-
Packaging Specification:
Available as film-coated oral tablets in strengths of 80 mg and 240 mg .
Storage:
Store at 20–25°C (68–77°F); excursions permitted between 15–30°C (59–86°F). Protect from light and moisture .
Expiry Date:
Refer to the expiration date printed on the packaging.
Executive Standard:
Manufactured in accordance with FDA-approved standards and guidelines.
Approval Number:
FDA approval date: August 19, 2024 .
Date of Revision:
October 1, 2024 .
Manufacturer:
Janssen Biotech, Inc.
Guidelines for the Use of Lazertinib
Dosage and Administration:
-
Recommended Dose: 240 mg orally once daily in combination with amivantamab.
-
Administration: Swallow tablets whole; do not crush, split, or chew. May be taken with or without food.
-
Missed Dose: If a dose is missed by less than 12 hours, take it as soon as possible. If more than 12 hours have passed, skip the missed dose and resume at the regular time.
-
Vomiting: If vomiting occurs after taking a dose, do not take an additional dose; take the next dose at the scheduled time .
Adverse Reactions:
Common adverse reactions (≥20%) include:
-
Rash
-
Nail toxicity
-
Infusion-related reactions (associated with amivantamab)
-
Musculoskeletal pain
-
Edema
-
Stomatitis
-
Venous thromboembolic events (VTE)
-
Paresthesia
-
Fatigue
-
Diarrhea
-
Constipation
-
Dry skin
-
Decreased appetite
-
Pruritus
-
Nausea
-
Ocular toxicity .
Contraindications:
No known contraindications have been identified .
Precautions:
-
Venous Thromboembolic Events (VTE): Serious and potentially fatal VTE, including deep vein thrombosis and pulmonary embolism, have occurred. Prophylactic anticoagulation is recommended for the first 4 months of treatment. Monitor for signs and symptoms of VTE and manage as clinically indicated.
-
Interstitial Lung Disease (ILD)/Pneumonitis: ILD or pneumonitis may occur. Monitor for new or worsening respiratory symptoms. If ILD/pneumonitis is suspected, withhold treatment and permanently discontinue if confirmed.
-
Dermatologic Toxicity: Severe skin reactions, including rash, pruritus, and dry skin, have been reported. Advise patients to use alcohol-free emollients and limit sun exposure. Manage skin reactions with topical corticosteroids and/or antibiotics as appropriate.
-
Ocular Toxicity: Ocular adverse reactions, including keratitis, may occur. Promptly refer patients with new or worsening eye symptoms to an ophthalmologist.
-
Embryo-Fetal Toxicity: Lazertinib may cause fetal harm. Advise females of reproductive potential to use effective contraception during treatment and for 3 weeks after the last dose. Males with female partners of reproductive potential should also use effective contraception during treatment and for 3 weeks after the last dose .
Lazertinib Interactions
Drug Interactions:
-
CYP3A4 Inducers: Avoid concomitant use with strong or moderate CYP3A4 inducers (e.g., rifampin, efavirenz) as they can significantly decrease lazertinib plasma concentrations, potentially reducing efficacy.
-
CYP3A4 Inhibitors: Concomitant use with strong CYP3A4 inhibitors (e.g., itraconazole) can increase lazertinib exposure; monitor for adverse reactions.
-
CYP3A4 Substrates: Lazertinib is a weak CYP3A4 inhibitor and may increase plasma concentrations of CYP3A4 substrates (e.g., midazolam); monitor for adverse reactions.
-
BCRP Substrates: Lazertinib is a BCRP inhibitor and may increase plasma concentrations of BCRP substrates (e.g., rosuvastatin); monitor for adverse reactions .
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.







Reviews
There are no reviews yet.