Ryoncil (Amimestrocel Injection) – SR-aGVHD | HongKong DengYue Medicine
- Generic Name/Brand Name: Amimestrocel/Ryoncil
- Indications: SR-aGVHD
- Dosage Form: Injection
- Specification: 3.8 ml × 1 vial
Amimestrocel Injection Application Scope
Amimestrocel is indicated for the treatment of steroid-refractory acute graft-versus-host disease (aGVHD) with predominant gastrointestinal involvement in patients aged 14 years and older.
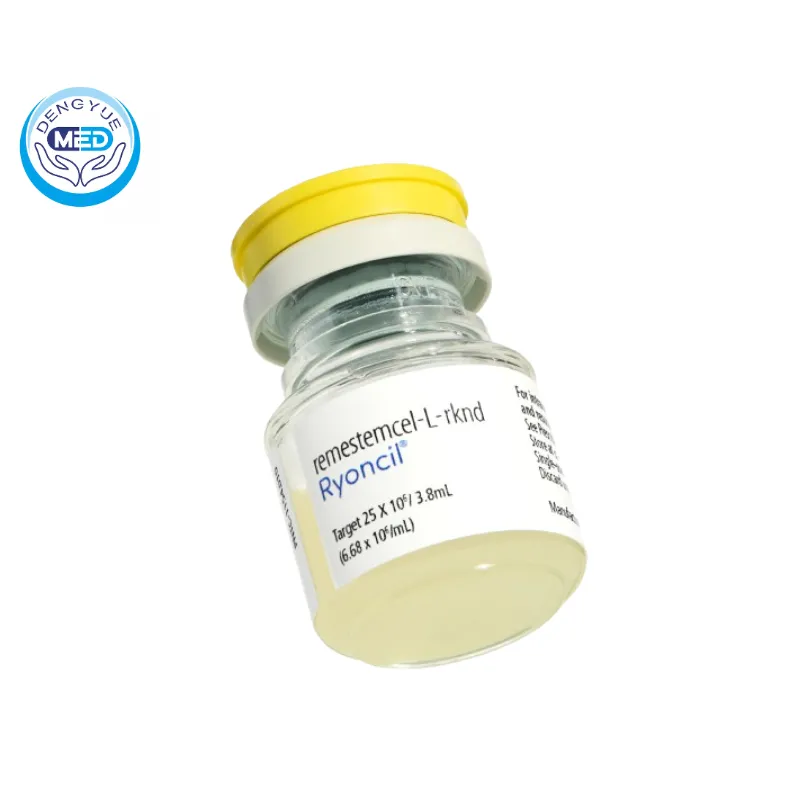
Amimestrocel Injection Characteristics
-
Ingredients: Amimestrocel (allogeneic mesenchymal stem cells / MSC-based therapy).
-
Properties: Colorless to slightly yellow clear liquid, free of foreign matter and precipitation.
-
Packaging Specification: 3.8 ml × 1 vial.
-
Storage: Store at 2-8°C under refrigeration, avoid freezing and exposure to light.
-
Expiry Date: As indicated on the label; stability depends on cryopreservation conditions.
-
Executive Standard: In accordance with relevant cell therapy GMP regulations and clinical guidelines.
-
Approval Number: As indicated on the packaging.
-
Date of Revision: As indicated on the packaging.
-
Manufacturer: As indicated on the packaging.
Guidelines for the Use of Amimestrocel Injection
-
Dosage and Administration:
-
Recommended Dose: Determined by the prescribing physician based on clinical trial protocol or treatment guidelines.推
-
Administration: Administered intravenously under medical supervision in a certified healthcare facility.
-
Missed Dose: If a scheduled infusion is missed, consult the prescribing physician immediately.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Transient fever, headache, chills, mild infusion-related reactions.
-
Serious Adverse Reactions: Rare; may include severe allergic reaction, immune response, or infection related to cell infusion.
-
-
Contraindications:
-
Known hypersensitivity to any excipients.
-
Active systemic infection.
-
Severe uncontrolled comorbidities as assessed by the physician.
-
-
Precautions:
-
Use only under the supervision of a qualified specialist.
-
Monitor patients closely during and after infusion.
-
Ensure proper screening of patients before administration.
-
Amimestrocel Injection Interactions
-
No confirmed drug–drug interactions established to date.
-
Caution is advised when combined with immunosuppressive or immunomodulatory therapies; physician evaluation is required.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.