Telitacicept Mechanism of Action—A Chinese Innovative “Dual-Target” Breakout
In the field of autoimmune disease treatment, 2021 marked a significant milestone—the world’s first-in-class dual-target biologic drug Telitacicept (brand name: Tai Ai®), independently developed in China, was officially approved for marketing for the treatment of systemic lupus erythematosus.
This not only signifies China’s leap from “following” to “keeping pace” in the research and development of high-end biologics but also heralds a new therapeutic paradigm.
Now, four years later, Telitacicept’s scope has expanded far beyond lupus. The Telitacicept mechanism of action—its unique dual-target design—is demonstrating powerful potential across one autoimmune disease area after another.
From IgA nephropathy to myasthenia gravis, this “dual-target breakthrough” continues to deliver exciting clinical data, revealing its immense potential to become a “best-in-class” therapy in multiple therapeutic fields.
👉 As a key global pharma partner for this drug in the Chinese market, HongKong DengYueMedicine is committed to delivering this innovative therapy to a broader patient population.
This article will provide an in-depth analysis of its distinctive mechanism of action and, based on the latest clinical trial data, comprehensively illustrate how this mechanism translates into groundbreaking clinical efficacy across different indications.
Core Mechanism: The “Source-Targeting” Strategy of Dual-Target Design
To understand why Telitacicept has demonstrated transformative potential across diverse autoimmune diseases, we must first examine the foundational science behind its design.
👉 The Telitacicept mechanism of action represents a paradigm shift from broad immunosuppression to precise, upstream immunomodulation.
✅ At the heart of its efficacy lies a sophisticated telitacicept structure engineered to address a common pathological driver shared by many autoimmune conditions.
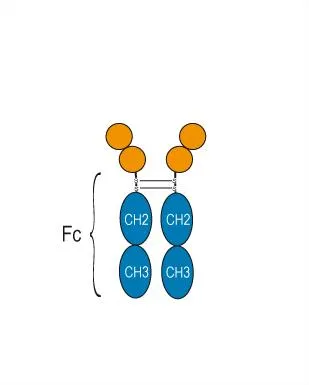
The Common Pathological Core of Autoimmune Diseases: Dysregulated B Cells and “Harmful Antibodies”
The shared feature of most autoimmune diseases is the abnormal activation of B lymphocytes, which differentiate into plasma cells and produce large quantities of autoantibodies (commonly referred to as “harmful antibodies”) that attack the body’s own tissues:
- Systemic Lupus Erythematosus (SLE): Produces anti-double-stranded DNA (dsDNA) antibodies, among others.
- IgA Nephropathy (IgAN): Produces galactose-deficient IgA1 (Gd-IgA1) antibodies.
- Myasthenia Gravis (gMG): Produces anti-acetylcholine receptor (AChR) antibodies.
- Neuromyelitis Optica Spectrum Disorder (NMOSD): Produces anti-aquaporin-4 (AQP4) antibodies.
🤔 Conventional treatments such as glucocorticoids and broad-spectrum immunosuppressants can suppress inflammation but act like a “carpet-bombing” approach—they come with significant side effects and fail to precisely intervene at the source of antibody production.
The Precision Targeting of Telitacicept: Simultaneously Neutralizing BLyS and APRIL
The telitacicept structure is that of a recombinant TACI-Fc fusion protein.
The ingenuity of its design lies in its ability to simultaneously target the two most critical cytokines for B-cell survival and maturation:
- B Lymphocyte Stimulator (BLyS, also known as BAFF)
- A Proliferation-Inducing Ligand (APRIL)
Detailed Explanation of the Telitacicept Mechanism of Action:
- “Intelligent Decoy” Strategy: The TACI domain of telitacicept functions as a “decoy receptor,” efficiently binding to and neutralizing excessive BLyS and APRIL in the bloodstream.
- Cutting Off “Survival Signals”: BLyS and APRIL are essential “survival signals” for B cells to survive, differentiate into plasma cells, and produce antibodies. When the neutralization rate exceeds a certain threshold (typically >60%), abnormally activated B cells undergo apoptosis due to “signal starvation.”
- Reducing Antibodies at the Source: With fewer plasma cell precursors, the production of pathogenic autoantibodies is significantly suppressed at its origin.
Synergistic Advantages of the Dual-Target Approach:
- Compared to single-target drugs that only inhibit BLyS (such as belimumab), simultaneously neutralizing both BLyS and APRIL more comprehensively blocks the B-cell pathway. This is particularly effective against IgA-producing B cells and long-lived plasma cells residing in the bone marrow.
- This explains why the telitacicept mechanism of action demonstrates particularly outstanding efficacy in diseases highly dependent on antibodies, such as IgAN and gMG.
This elegant and precise telitacicept mechanism of action provides the robust scientific foundation for its clinical success.
By strategically dismantling the core support system for pathogenic B cells, telitacicept achieves what conventional therapies cannot: targeted intervention at the disease’s origin.
Now that we understand how it works, let us examine what it has achieved.
The following section will present the compelling clinical evidence—the remarkable outcomes and hard data—that demonstrate how this mechanism translates into transformative results across its four major indications.
Clinical Triumphs: Latest Advancements and Robust Data Across Four Major Indications
The compelling theoretical foundation of telitacicept’s mechanism has been powerfully validated in the clinical arena.
Moving from bench to bedside, it has delivered a series of landmark results across diverse autoimmune diseases, redefining treatment expectations.
🔻 The following data showcase not just efficacy but the tangible impact of precision immunomodulation on patient outcomes.
Systemic Lupus Erythematosus (SLE)—The Foundational Victory
✅ The first and currently only dual-target biologic approved for SLE in China.
Pivotal Phase III Data (Published in Ann Rheum Dis):
- At Week 52, 82.6% of patients in the telitacicept group achieved the SLE Responder Index 4 (SRI-4), significantly higher than 38.1% in the placebo group.
- Higher seroconversion rates were observed in anti-dsDNA antibody-positive patients.
- Significant steroid-sparing effect: Enabled dose reduction or discontinuation of corticosteroids for many patients.
👉 Provides an effective and precise new option for patients with moderate-to-severe SLE who have an inadequate response to conventional therapies.
IgA Nephropathy (IgAN)—A Disruptive Breakthrough in Nephrology
✅ The Phase III trial met its primary endpoint in 2023. A new drug application for this indication has been submitted to China’s NMPA, with approval imminent.
Key Phase III Data Highlights:
- Primary Endpoint (Proteinuria): After 39 weeks, the 24-hour urine protein-to-creatinine ratio (24h-UPCR) decreased by 58.9% from baseline in the Telitacicept group versus only 8.8% in the placebo group (P < 0.0001).
- Deep Remission Rates:
- 61% of patients achieved UPCR < 0.8 g/g (low-risk threshold), compared to 19.5% in the placebo group.
- 42.1% achieved deep remission (UPCR < 0.5 g/g), versus 7.5% with placebo.
- Renal Function Protection: The estimated glomerular filtration rate (eGFR) slope remained stable in the Telitacicept group (-0.010 mL/min/1.73 m²/year), while it declined significantly in the placebo group (-0.77 mL/min/1.73 m²/year), demonstrating effective slowing of renal function decline.
- Safety Surprise: The incidence of serious adverse events was lower in the treatment group (2.5%) than in the placebo group (8.2%).
The data perfectly validate the hypothesis that by inhibiting BLyS/APRIL and reducing pathogenic Gd-IgA1 production, Telitacicept treats IgAN at its source.
Myasthenia Gravis (gMG)—Potential “Best-in-Class” Breakthrough Data
✅ In April 2024, its China Phase III data were presented as a “Late-Breaking” oral presentation at the American Academy of Neurology (AAN) Annual Meeting, capturing significant industry attention.
Phase III Data Interpretation:
- Primary Endpoint (MG-ADL improvement ≥3 points): After 24 weeks, the response rate was 98.1% in the Telitacicept group versus only 12% in the placebo group—an unprecedented result in global gMG Phase III studies.
- Benchmark Comparison:
- Eculizumab (C5 inhibitor) REGAIN study (26-week): 60% vs 34%.
- Efgartigimod (FcRn antagonist) ADAPT study (improvement ≥2 points): 67.7% vs 29.7%.
- Rapid Onset: A statistically significant difference versus placebo emerged as early as Week 4.
- Greater Efficacy in More Severe Disease: The baseline MG-ADL score in this study was high at 10 points (scale of 0-24), exceeding the baseline scores of the two benchmark trials (6.3 and 8.5 points), proving its exceptional efficacy even in a more severely affected population.
- Safety Redefines Expectations:
- Infections: Incidence was lower in the treatment group (45.6%) than in the placebo group (59.6%).
- Serious Adverse Events (SAEs): The Telitacicept group had a lower SAE rate, with significantly fewer respiratory-related SAEs—a crucial factor for MG patients prone to respiratory crises.
With a near-100% response rate, rapid onset of action, and an excellent safety profile, Telitacicept demonstrates clear best-in-class potential in gMG.
Neuromyelitis Optica Spectrum Disorder (NMOSD)—Preventing Relapse
✅ Already approved in China for adult patients with AQP4 antibody-positive NMOSD.
Phase III studies showed that Telitacicept significantly reduces the annualized relapse rate and prolongs the time to relapse, offering an important additional weapon beyond traditional immunosuppressants and complement inhibitors.
This reaffirms that inhibiting the B-cell pathway and reducing anti-AQP4 antibody production is an effective strategy for preventing NMOSD relapses.
✨ The clinical achievements of telitacicept across SLE, IgAN, gMG, and NMOSD form a cohesive and powerful narrative.
As research continues, the reach of this innovative mechanism may well extend beyond these four indications, heralding a new era of targeted therapy for a broader range of autoimmune conditions.
Safety Profile and Future Outlook: A Rational Perspective on Broad Prospects
The true value of any innovative therapy lies in the balance between efficacy and safety.
As Telitacicept demonstrates remarkable clinical benefits, its safety characteristics and long-term application prospects have become focal points for both the medical community and patients.
Overall Safety Profile
The safety of Telitacicept is intrinsically linked to its precise mechanism of B-cell pathway inhibition.
It is generally well-tolerated, with most adverse reactions being mild to moderate in severity.
- Common Adverse Reactions: Primarily include mild to moderate upper respiratory tract infections (e.g., nasopharyngitis) and injection site reactions (e.g., redness, pain).👉 These are typically transient and manageable.
- Critical Monitoring and Contraindications:
- Contraindicated in Active Infections: Due to its immunomodulatory effects, active infections such as tuberculosis, hepatitis B, and hepatitis C must be screened for and ruled out prior to initiation.
- Immunoglobulin Monitoring: Some patients may experience reversible decreases in immunoglobulin levels (particularly IgG, IgA, and IgM), necessitating regular monitoring to ensure levels remain within a safe range.
- Vaccination: Administration of live vaccines should be avoided during treatment.
- Safety Data Highlights: The incidence of serious adverse events in the Telitacicept treatment groups was no higher, and in some cases lower, than in the placebo groups.
✨ These findings challenge conventional expectations and suggest that the substantial clinical benefit resulting from potent disease control may significantly offset, or even outweigh, potential immunosuppression-related risks, thereby providing important confidence for long-term treatment.
Future Outlook and Challenges
The success of Telitacicept extends beyond its current achievements, encompassing future possibilities and practical challenges.
1️⃣ Expansion of Indications:
Its potential as a “one-drug, multiple-disease” therapy is under active exploration.
👉 Clinical studies for other B-cell-mediated autoimmune diseases, such as Primary Sjögren’s Syndrome, Multiple Sclerosis, and Pemphigus, are underway, promising to further unlock its therapeutic value.
2️⃣ Globalization Journey:
As a First-in-class innovative drug originating from China, Telitacicept’s international multicenter clinical trials are progressing steadily.
👉 These aim to validate its efficacy and safety across diverse populations, ultimately benefiting patients worldwide and marking a critical step for Chinese innovative drugs on the global stage.
3️⃣ Accessibility and Reimbursement:
As more indications gain approval, efforts to include Telitacicept in national and regional medical insurance catalogs are crucial.
👉 Improving drug accessibility and alleviating the economic burden on patients are key to realizing its broader societal value.
4️⃣ Clinical Decision-Making and Precision Use:
Telitacicept is a prescription medication.
👉 Its initiation, dose adjustment, and long-term management must be carefully decided by specialists in rheumatology, nephrology, or neurology, based on a comprehensive assessment of the patient’s specific condition, autoantibody profile, infection risk, and comorbidities, to achieve personalized and precise treatment.
In summary, the safety profile of Telitacicept is well-defined and manageable within the context of its precise mechanism of action. The observed trend of “improved safety driven by superior efficacy” is particularly valuable.
Conclusion
The development journey of Telitacicept exemplifies the paradigm of translating precise scientific discovery—its dual-target mechanism—into solutions for multiple critical clinical needs.
It has not only achieved a breakthrough from 0 to 1 in the field of systemic lupus erythematosus but has also produced potentially transformative data in IgA nephropathy and myasthenia gravis, moving from 1 to N with its superior “source-targeting” therapeutic approach.
This success extends far beyond the triumph of a single drug; it epitomizes the growing capabilities of China’s biopharmaceutical innovation ecosystem.
It demonstrates that Chinese scientists and pharmaceutical companies are capable of proposing original insights at the forefront of target science and drug design, and of translating these insights through rigorous clinical research into blockbuster therapies that benefit patients worldwide.
From SLE to IgAN and then to gMG, Telitacicept is steadily fulfilling its promise as a “transformative therapy” along its scientific trajectory.
In the future, it will continue to serve as a precise “immune key,” opening the door to long-term remission and a high-quality life for more patients struggling with autoimmune diseases.
DengYue Medicine remains dedicated to delivering cutting-edge therapeutic solutions and authoritative drug information to advance patient care and clinical practice.
FAQ about Telitacicept Mechanism of Action
What is Telitacicept?
Telitacicept (Tai’ai®) is fusion protein comprising a recombinant transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) receptor fused to the fragment crystallizable (Fc) domain of human immunoglobulin G (IgG).
What is the mechanism of action of Telitacicept?
Tocilizumab binds soluble and membrane bound IL-6 receptors, preventing IL-6 mediated inflammation.
How is Telitacicept administered?
Telitacicept is administered via subcutaneous injection, which makes it relatively easy for patients to use.
Is Telitacicept a monoclonal antibody?
The design of telitacicept is distinctive; it is not a monoclonal antibody but a TACI-Fc fusion protein generated through recombinant DNA technology.