Xphozah (Tenapanor Hydrochloride) – IBS-C | HongKong DengYue Medicine
- Generic Name/Brand Name: Tenapanor/Xphozah
- Indications: IBS-C
- Dosage Form: Tablets
- Specification: 50mg x 60
Tenapanor Hydrochloride Application Scope
Tenapanor is indicated for the treatment of irritable bowel syndrome with constipation (IBS-C) in adults.
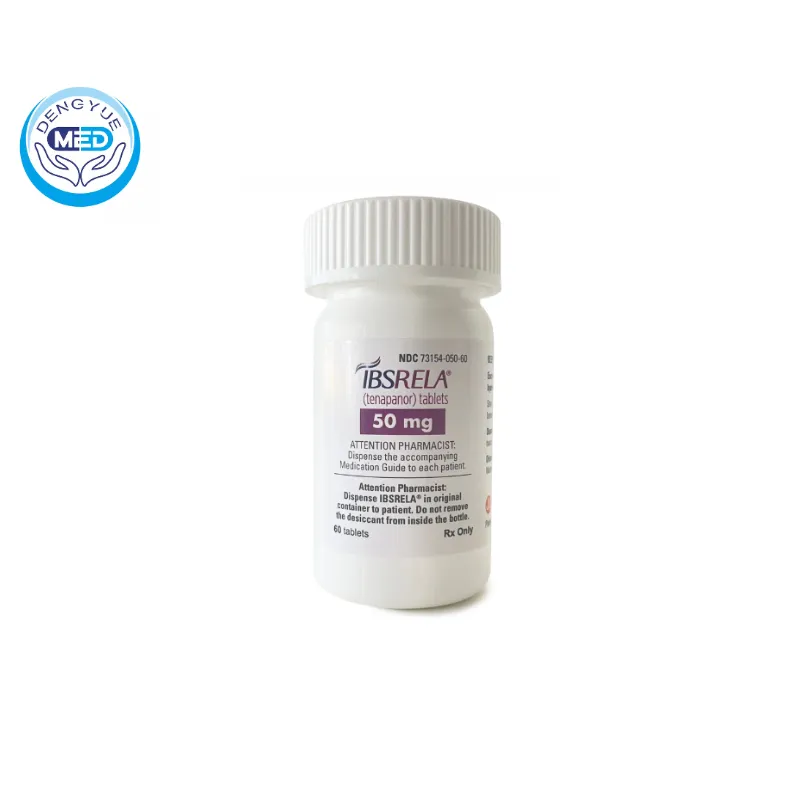
Tenapanor Hydrochloride Characteristics
-
Ingredients: Active ingredient: Tenapanor hydrochloride
-
Properties: White to off-white powder; poorly soluble in water; acts locally in the gastrointestinal tract.
-
Packaging Specification: As indicated on the package.
-
Storage: Store at room temperature (20–25 °C), protect from moisture and direct light.
-
Expiry Date: As indicated on the package.
-
Executive Standard: As indicated on the package.
-
Approval Number: As indicated on the package.
-
Date of Revision: As indicated on the package.
-
Manufacturer: As indicated on the package.
Guidelines for the Use of Tenapanor Hydrochloride
-
Dosage and Administration:
-
Recommended Dose: 50 mg orally, twice daily.
-
Administration: Take immediately before the morning and evening meals.
-
Missed Dose: If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not double the dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Diarrhea, abdominal distension, flatulence, dizziness.
-
Serious Adverse Reactions: Severe diarrhea (may lead to dehydration). Discontinue and seek medical attention if severe diarrhea occurs.
-
-
Contraindications:
-
Known hypersensitivity to Tenapanor or any excipients.
-
Children under 6 years of age (risk of severe dehydration).
-
Avoid use in patients with suspected or known mechanical gastrointestinal obstruction.
-
-
Precautions:
-
Safety not established in pediatric patients under 18 years.
-
Use with caution in elderly or patients with severe diarrhea.
-
Monitor hydration status in patients at risk of dehydration.
-
Tenapanor Hydrochloride Interactions
-
Minimal systemic absorption; drug–drug interactions are not common.
-
Avoid concurrent use with medications that can cause diarrhea.
-
Consult a physician before use with other IBS-C therapies.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.