Lenvima (Lenvatinib) – HCC | HongKong DengYue Medicine
- Generic Name/Brand Name: Lenvatinib / Lenvima®
- Indications: Thyroid, kidney, liver, and endometrial cancers
- Dosage Form: Oral capsules
- Specification: 4 mg, 10 mg, 20 mg × 28 capsules/box
Lenvatinib Application Scope
• Differentiated thyroid cancer (DTC) refractory to radioactive iodine therapy
• Advanced renal cell carcinoma (RCC) in combination with everolimus
• Unresectable hepatocellular carcinoma (HCC)
• Advanced endometrial carcinoma in combination with pembrolizumab
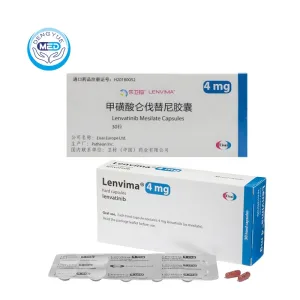
Lenvatinib Characteristics
-
Ingredients: Lenvatinib mesylate
-
Properties: Oral, small molecule multi-kinase inhibitor
-
Packaging Specification: 4 mg, 10 mg, or 20 mg capsules; typically packaged as 28 capsules per box
-
Storage: Store at room temperature, 20°C to 25°C (68°F to 77°F). Protect from moisture and light.
-
Expiry Date: See package labeling (generally 24 months)
-
Executive Standard: Compliant with FDA and EMA regulations
-
Approval Number: Example (U.S.): NDA 208573
-
Date of Revision: August 2025 (or latest available)
-
Manufacturer: Eisai Co., Ltd.
Guidelines for the Use of Lenvima
-
Dosage and Administration:
-
Recommended Dose:
-
Differentiated thyroid cancer: 24 mg orally once daily
-
Renal cell carcinoma: 18 mg orally once daily (in combination with everolimus)
-
Hepatocellular carcinoma: 12 mg (bodyweight ≥60 kg) or 8 mg (bodyweight <60 kg) orally once daily
-
Endometrial carcinoma: 20 mg orally once daily (in combination with pembrolizumab)
-
-
Administration: Swallow capsules whole with water, with or without food. Take at the same time daily.
-
Missed Dose: If a dose is missed, take as soon as possible on the same day. Do not double dose the next day.
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5% and >3% higher than placebo):
-
-
Hypertension
-
Diarrhea
-
Fatigue
-
Decreased appetite
-
Nausea
-
Weight loss
-
Proteinuria
-
Palmar-plantar erythrodysesthesia syndrome (hand-foot syndrome)
-
-
-
Serious Adverse Reactions:
-
-
Hepatotoxicity
-
Cardiac dysfunction (including heart failure)
-
Gastrointestinal perforation or fistula
-
Severe hypertension
-
Hemorrhage
-
-
-
-
Contraindications:Hypersensitivity to lenvatinib or any component of the formulation
-
Precautions:
-
-
Hypertension:
Monitor blood pressure regularly and manage accordingly. -
Hepatic Impairment:
Use with caution in patients with liver dysfunction. -
Cardiac Function:
Monitor for signs of cardiac dysfunction. -
Pregnancy and Lactation:
Not recommended during pregnancy; advise effective contraception. Unknown if excreted in breast milk; avoid breastfeeding.
-
-
Lenvatinib Interactions
-
CYP3A4 Inducers/Inhibitors:
Co-administration may affect lenvatinib plasma levels; monitor for efficacy and toxicity. -
Anticoagulants:
Use caution due to increased bleeding risk. -
Other Tyrosine Kinase Inhibitors or Anti-hypertensives:
Monitor for additive adverse effects.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.







Reviews
There are no reviews yet.