Firsekibart Injection Approved by NMPA: A Breakthrough IL-1β Antibody Offering Long-Acting Relief for Acute Gouty Arthritis
The National Medical Products Administration (NMPA) has officially approved Firsekibart Injection, a Class 1 innovative biologic drug independently developed by Changchun GeneScience Pharmaceuticals Co., Ltd.
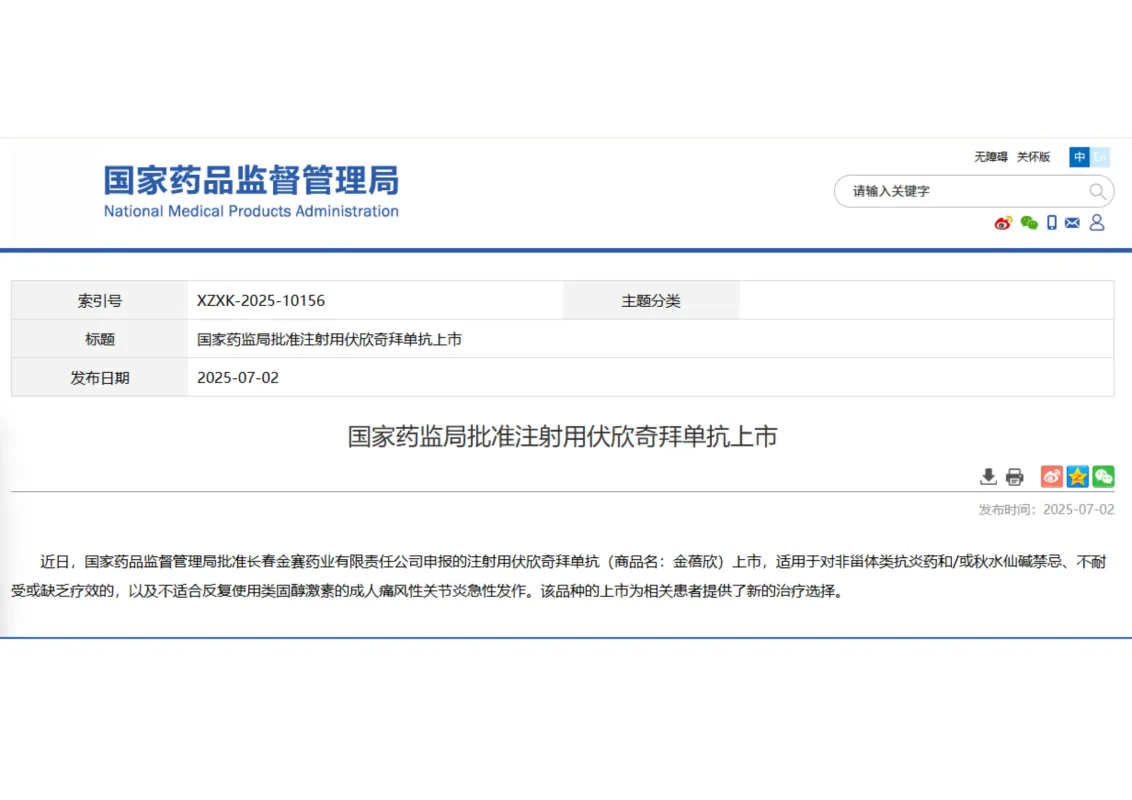
✨ This approval marks a major milestone in China’s treatment landscape for acute gouty arthritis, a painful and recurrent inflammatory disease that affects millions of adults.
💉 Firsekibart injection is indicated for adult patients with acute gouty arthritis who are contraindicated, intolerant, or unresponsive to nonsteroidal anti-inflammatory drugs (NSAIDs) and/or colchicine, as well as those unsuitable for repeated use of corticosteroids.
💡 With this approval, GeneScience Pharmaceuticals brings to market China’s first IL-1β monoclonal antibody, providing an innovative, long-acting, and safe biologic therapy option for patients with limited treatment alternatives.
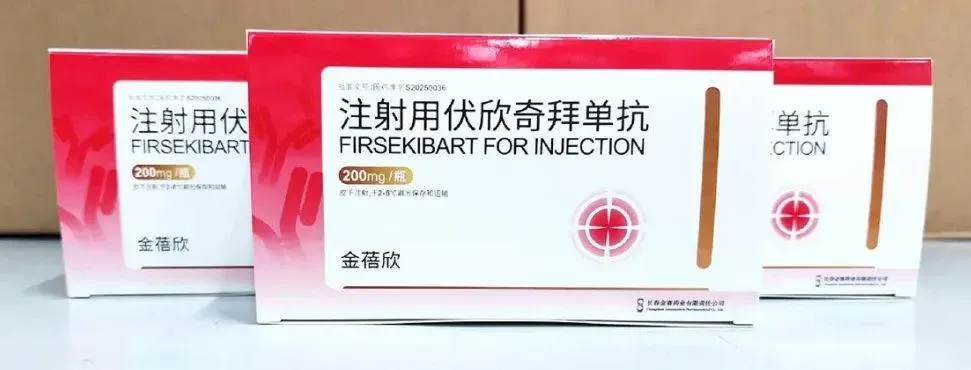
Redefining Gout Management Through Innovation
Firsekibart represents a first-in-class breakthrough in the treatment of gout and inflammation-driven diseases.
👉 It is the world’s first fully human IgG4/λ subtype anti-IL-1β monoclonal antibody, developed to selectively and precisely inhibit the IL-1β signaling pathway—the key driver of gout inflammation.
🫷 By blocking IL-1β binding to its receptor, Firsekibart suppresses the downstream inflammatory cascade triggered by monosodium urate crystals that accumulate in the joints.
🌟 This targeted mechanism allows Firsekibart to control inflammation at its origin, offering rapid pain relief and long-term prevention of recurrent flares.
Pharmacokinetic studies show that Firsekibart has an extended half-life of 25.5 to 30.8 days, supporting a “two injections per year” dosing regimen
🌟 A remarkable improvement in convenience and compliance for patients suffering from chronic and recurrent gout.
Robust Clinical Data Validates Long-Acting Efficacy
The NMPA approval was supported by results from a Phase III multicenter, randomized, double-blind, positive-controlled clinical trial involving 313 patients with acute gouty arthritis.
✅ The study comprehensively evaluated Firsekibart’s efficacy, safety, and tolerability compared with compound betamethasone.
Key Clinical Outcomes
- 🔴Rapid pain relief: Firsekibart demonstrated comparable pain reduction to compound betamethasone within 6 to 72 hours post-administration, meeting the pre-specified non-inferiority criteria.
- 🟠Superior recurrence prevention: Within 12 weeks after a single injection, the median time to first flare was not reached in the Firsekibart group, compared to 45 days in the control group—representing a 90% reduction in recurrence risk.
- 🟡Sustained control: Over 24 weeks, Firsekibart further reduced the first recurrence risk by 87%, and 85.3% of patients experienced zero relapse.
- 🟢Excellent safety profile: No treatment-related serious adverse events (SAEs) were reported in the Firsekibart arm, while three SAEs occurred in the betamethasone group.
Overall, the drug was well tolerated, demonstrating better safety than traditional corticosteroids.
These findings confirm that Firsekibart offers rapid symptom control, prolonged remission, and outstanding tolerability, establishing a new benchmark for biologic therapy in gout management.
A Convenient New Formulation for Clinical Practice
Building on its success with the lyophilized formulation, GeneScience Pharmaceuticals developed a ready-to-use Firsekibart injection to further improve clinical convenience.
💉 The new liquid formulation eliminates the need for reconstitution or dilution before administration, streamlining treatment in both hospital and outpatient settings.
In a bioequivalence study conducted in healthy Chinese male volunteers, the injection demonstrated equivalent pharmacokinetic and safety profiles compared to the lyophilized version.
This advancement enhances usability for healthcare professionals and improves patient accessibility, reflecting GeneScience’s commitment to continuous pharmaceutical innovation and patient-centered design.
Addressing an Urgent Unmet Need in Gout Treatment
🦵 Gouty arthritis is a chronic metabolic disease characterized by intense joint inflammation and severe pain caused by uric acid crystal deposition.
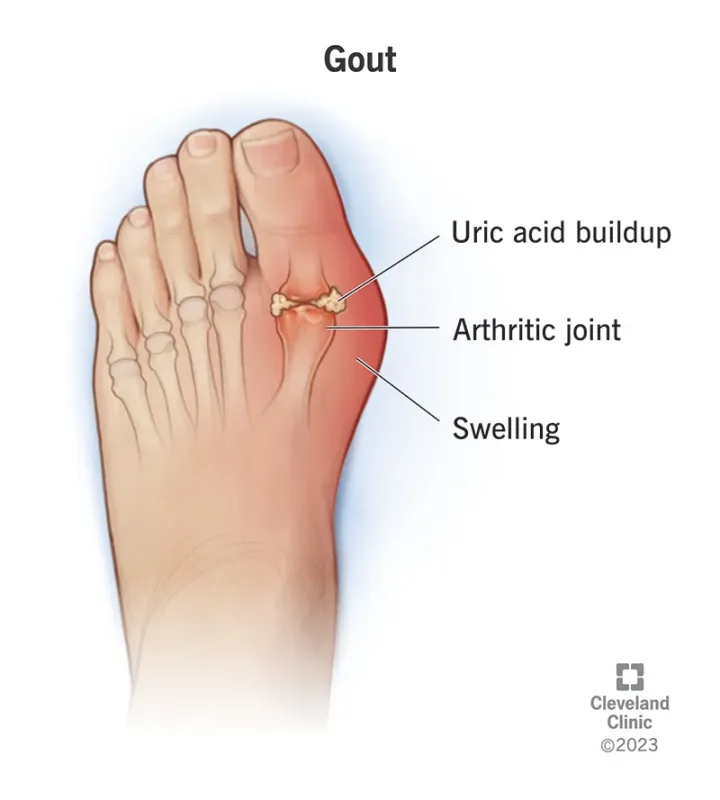
❗ According to the 2021 China Hyperuricemia and Gout Trend White Paper, approximately 177 million Chinese adults suffer from hyperuricemia, and over 14.6 million patients live with gouty arthritis, making it the second most prevalent metabolic disorder after diabetes.
😟 Despite widespread use of NSAIDs, colchicine, and corticosteroids, up to 50% of patients fail to achieve adequate symptom control, and nearly 60% experience recurrent flares within a year.🔻
Existing treatments often provide only short-term relief and may cause gastrointestinal or cardiovascular side effects, leaving many patients in need of safer and more durable anti-inflammatory options.
🙂 Firsekibart injection directly addresses this unmet clinical need with its targeted IL-1β inhibition, prolonged half-life, and excellent safety, offering a transformative solution for patients who cannot tolerate or do not respond to conventional therapies.
Market Outlook: A Promising Future for Firsekibart Injection
💰 China’s gout drug market is projected to reach 10.8 billion RMB (approximately USD 1.5 billion) by 2030, driven by rising disease prevalence, unmet treatment needs, and growing acceptance of biologic therapies.
👉 With its unique mechanism, superior efficacy, and convenient long-acting dosing, Firsekibart is poised to become a key player in China’s gout therapeutics landscape.
In addition, the distribution of Firsekibart injection in Hong Kong and international markets will benefit from partnerships with trusted and certified distributors such as Hong Kong DengYue Medicine, a Hong Kong Department of Health–licensed pharmaceutical distributor.

☀️ Their expertise in importing, warehousing, and distributing innovative drugs ensures patients have timely access to Firsekibart injection, further supporting the drug’s market penetration and patient accessibility.
Jinbeixin (Firsekibart) – Gouty Arthritis (GA) | HongKong DengYue Medicine
- Generic Name/Brand Name: Firsekibart/Jinbeixin
- Indications: Gouty Arthritis (GA)
- Dosage Form: Freeze-dried powder injection
- Specification: 200 mg × 1 vial



