Xeljanz (Tofacitinib) – Rheumatoid Arthritis | HongKong DengYue Medicine
- Generic Name/Brand Name: Tofacitinib / Xeljanz®
- Indications: RA, PsA, UC
- Dosage Form: Oral tablets
- Specification: 5 mg, 10 mg × 60 tablets/box
Tofacitinib Application Scope
Indicated for the treatment of moderate-to-severe active rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis in adult patients who have had an inadequate response or intolerance to one or more DMARDs (disease-modifying antirheumatic drugs).
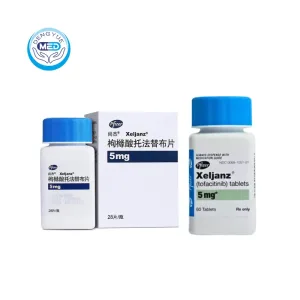
Tofacitinib Characteristics
-
Ingredients: Tofacitinib
-
Properties: Janus kinase (JAK) inhibitor, small molecule, orally administered
-
Packaging Specification: 5 mg and 10 mg film-coated tablets; blister packs or bottles
-
Storage: Store below 25°C in a dry place, protected from light and moisture
-
Expiry Date: 24 months from the date of manufacture
-
Executive Standard: In accordance with pharmacopeia standards and regulatory approval requirements
-
Approval Number: NDA 203214 S-038
-
Date of Revision: August 23, 2024
-
Manufacturer: Pfizer Inc.
Guidelines for the Use of Xeljanz
-
Dosage and Administration:
-
Administration: Take with or without food; swallow whole, do not crush or split tablets
-
Missed Dose: If a dose is missed, take as soon as possible; resume regular schedule
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5% and >3% higher than placebo):
-
-
Upper respiratory tract infections
-
Nasopharyngitis
-
Diarrhea
-
Headache
-
Hypertension
-
-
-
Serious Adverse Reactions:
-
-
Serious infections (tuberculosis, invasive fungal infections, opportunistic infections)
-
Thrombosis (DVT, PE, arterial thrombosis)
-
Malignancies (lymphoma and other cancers)
-
-
-
-
Contraindications:
-
Patients with known hypersensitivity to Xeljanz or any excipient
-
Patients with active, serious infections
-
-
Precautions:
-
-
Infections: Monitor for signs of infection; interrupt therapy if serious infection develops
-
Blood Counts: Monitor lymphocyte counts, neutrophil counts, and hemoglobin before and during treatment
-
Liver Function: Monitor liver enzymes periodically
-
Pregnancy and Lactation: Use during pregnancy only if clearly needed; breastfeeding is not recommended during and for 18 hours after the last dose
-
-
Tofacitinib Interactions
-
Strong CYP3A4 inhibitors (e.g., ketoconazole): May increase Xeljanz exposure; reduce dose accordingly
-
Strong CYP3A4 inducers (e.g., rifampin): May decrease Xeljanz exposure; avoid co-administration
-
Immunosuppressants (e.g., cyclosporine, azathioprine): Increased risk of immunosuppression—avoid combination
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.