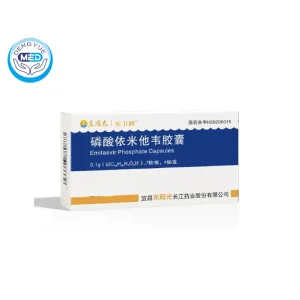
Emitasvir | Chronic Hepatitis C Virus (HCV) Infection | DengYue
- Generic Name/Brand Name: Emitasvir
- Indications: Chronic hepatitis C virus (HCV) infection
- Dosage Form: Oral film-coated tablets
- Specification: 0.1g, 28 tablets (7 tablets × 4 blisters) per box
Application Scope
Emitasvir is an NS5A inhibitor used in combination with Sofosbuvir for the treatment of chronic hepatitis C virus (HCV) infection in adults. It is effective against HCV genotypes 1 through 6, including those with compensated cirrhosis.
Characteristics
-
Ingredients: Emitasvir phosphate (the prodrug form of emitasvir)
-
Properties: Emitasvir is an orally active antiviral agent that inhibits the HCV NS5A protein, which is crucial for viral replication and assembly.
-
Packaging Specification: Typically available as film-coated tablets containing 0.1g
-
Storage: Store at room temperature, away from moisture and heat.
-
Expiry Date: Refer to the product packaging for the specific expiry date.
-
Executive Standard: Manufactured in accordance with Good Manufacturing Practices (GMP) and local regulatory standards.
-
Approval Number: Specific approval numbers vary by country; please consult local regulatory authorities.
-
Date of Revision: Refer to the product packaging for the date of the latest revision.
-
Manufacturer: Emitasvir is developed by Gilead Sciences, a biopharmaceutical company.
Guidelines for the Use of Emitasvir
-
Dosage and Administration:
-
Recommended Dosage: 200 mg of emitasvir once daily, co-administered with 400 mg of sofosbuvir.
-
Treatment Duration: Typically 12 weeks for treatment-naïve patients without cirrhosis or with compensated cirrhosis.
-
Administration: Taken orally, with or without food.
-
-
Adverse Reactions:
-
Common Side Effects: Headache, fatigue, nausea.
-
Serious Adverse Events: Elevated liver enzymes, bradycardia (when co-administered with sofosbuvir), and hypersensitivity reactions.
-
-
Contraindications:
-
Absolute Contraindications: Co-administration with strong inducers of P-glycoprotein (e.g., rifampin, St. John’s wort) is contraindicated.
-
Relative Contraindications: Use during pregnancy and breastfeeding is not recommended; effective contraception should be used during treatment.
-
-
Precautions:
-
Liver Function Monitoring: Regular monitoring of liver function tests is recommended during treatment.
-
Drug Interactions: Be cautious when co-administering with other medications metabolized by cytochrome P450 enzymes.
-
Interactions
- Drug Interactions:
-
Contraindicated Drugs: Strong P-glycoprotein inducers (e.g., rifampin, St. John’s wort).
-
Caution Advised: Other drugs metabolized by cytochrome P450 enzymes; consult with a healthcare provider before co-administering.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.